Drew was one of “those” patients.
Drew Hughes was an active, vibrant 13-year-old boy from North Carolina. In June of 2013, Drew was out skateboarding with his friends and fell backward, striking his head on the pavement. After visiting the local emergency department, the decision was made to transport Drew to the Level I Trauma Center. He was alert and conscious in the Emergency Department, but as a safety precaution the medical staff recommended Drew be sedated and a life sustaining breathing tube be placed in his airway for the long transport. En route, Drew woke up in the ambulance and removed the breathing tube from his airway–an event called unplanned extubation. The paramedics responded by injecting Drew with a medication to paralyze him so they could replace the breathing tube before continuing the transport. Over the next 30 minutes, Drew’s oxygen levels fell dramatically because his breathing tube had been improperly positioned in his esophagus rather than his trachea. As a result, Drew lost his life. If Drew’s breathing tube had been adequately stabilized, this preventable tragedy would never had occurred.
Unfortunately, Drew’s story isn’t unique. Every year, more than 33,000 people die from unplanned extubation.
We believe one death is one too many.
The problem is Unplanned Extubation: the accidental removal of a patient's life-sustaining breathing tube.

How is this acceptable as the standard of care?
1.65M
Intubated patients each year
36k
Patients will develop pneumonia
$4.9B
Wasted healthcare dollars
7.3%
Patients experience a UE
121k
Patients affected annually
33k
Patients die each year
In health care, a significant threat to ventilated patient safety is Unplanned Extubation, which occurs when a patient or other external force pulls an inadequately stabilized breathing tube out of the airway. Every year, Unplanned Extubation causes 33,000 preventable deaths, 121,000 life-threatening events, and racks up over $4.9 billion in wasteful healthcare spending. The median incidence rate of Unplanned Extubation is 7.3% in all ventilated ICU patients. As the current standard of care, this is unacceptable.
Our goal is to completely eliminate Unplanned Extubation and the problems associated with it, strengthening healthcare worldwide.
We have the solution.
It's called SolidAIRity.

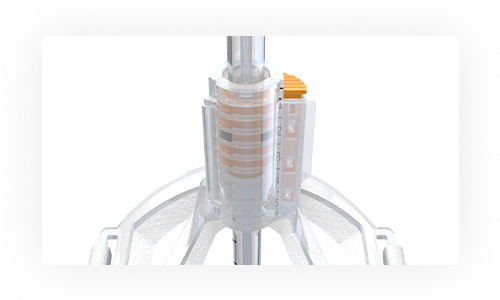
SolidAIRity is the only fully integrated airway stabilization system designed and patented to provide unmatched airway stability for ventilated patients. It’s unique ribbed endotracheal tube and interlocking stabilizer stands up to significantly more force than the leading competition, while maintaining unrestricted air flow. Competitor devices pinch, squeeze, or adhese to the breathing tube, decreasing air flow by as much as 40%. According to independent testing by biomedical engineers, SolidAIRity is so secure, the breathing tube will break before our system hits its fail point.
The First & Only
SolidAIRity™ is the only interlocking tube securement system. Competitor tube holders pinch, squeeze, or adhere to a smooth, slippery breathing tube.
Easy to Learn & Use
In independent testing by biomedical engineers, SolidAIRity™ restrains against 6.7 times more force than the closest competition.*
Unmatched Strength & Stability
The intuitive latching door simplifies securement and the tube is placed with the same intubation procedure qualified clinicians are used to.
A convergence of national data points to a 'perfect storm' in the market.

Strong Recognition
of the Problem
Significant Interest
in Disruptive Solution
High Likelihood of Acceptance
53%
Hospital administrator share of preference at highest price tested
33%
Clinician share of preference at highest price tested
75.12M endotracheal tubes sold annually worldwide
$4.9B spent on complications of inadequately secured airways in US ICU’s
Millions of patients all over the world are counting on us to get it right.
And an aging population along with increased incidence in chronic respiratory disease are expected to grow that number.
56%
projected growth in geriatric population by 2030
3rd
leading cause of death chronic respiratory disease
53%
projected growth tube & securement revenue
Since the competition hasn't solved the problem.
We will.

Because of our efforts, awareness and recognition of the problem is exploding.

These leading clinical, safety/risk and performance improvement organizations have committed to a national collaborative for driving increased awareness, data capture and best practice sharing to improve unplanned extubation rates.
We are partnering with clinically sophisticated and widely-reknowned healthcare leaders for early adoption and clinical data collection.

The hurdles in front of us are few and far between now.

Those we have cleared:
7.5M seed capital raised
Bench & voice of customer data collected
3rd Generation design optimized
3 patents issued
The remaining few:
FDA 510(k)
Post-clearance studies & limited market release
5 patents pending
Strategic partnership or exit
*Average clearance time frame for medical device traditional 510(k) applications reviewed by the US Food and Drug Administration in 2016 was 177 days based on data published by Emergo.
We are Securisyn.
Proven. Passionate. Committed.

Our Team
We are a proven, passionate, and committed commercialization team with strong clinical, industry and business backing from key opinion leaders. Our collective focus, experience and will to win strongly position us to become the industry leader for airway safety and device securement with the most recognized and favored solution in prevention of Unplanned Extubation.
Arthur Kanowitz, MD
Co-Founder & CMO
Invented SolidAIRity and has earned wide-spread recognition as the nation’s leading expert on Unplanned Extubation.
Served a gubernatorial appointment as the Inaugural CO State Emergency Medical and Trauma Services (EMTS) Medical Director.
Is diversely published in the medical literature.
Elyse Blazevich
Co-Founder, CFO & COO
Co-founded Securisyn and has successfully led the company from concept to market ready.
Has gained a strong reputation in the CO Bioscience community for her business acumen & industry knowledge.
Backed by a Master’s in Organizational Leadership with a specialization in Applied Business Management.
Mark Bruning
President & CEO
Significantly grew a PE owned healthcare company in 26 months resulting in a successful exit at an attractive multiple.
Led American Medical Response, the nation's largest ambulance provider serving over 4M patients annually.
Earned an MBA from Kellogg School of Management at Northwestern University.
Our Board Leaders
Bob DiSilvio
President, S-Force LLC
Rick Jory
President & CEO, Sandhill Scientific (Retired)
Gerry Lewis-Jenkins
Chief Operating Officer, COPIC
Bruno Darre
Chairman and CEO, 73 Holdings, Inc
We'd love to hear from you
For additional information, please use the form below or contact:
Elyse Blazevich, COO & CFO
eblaze@securisyn.com
303.594.6895
Securisyn Medical
9150 Commere Center Circle
Suite 135
Highlands Ranch, CO 80129
References:
1. Moons, P., et al., Development of a risk assessment tool for deliberate self-extubation in intensive care patients. Intensive Care Med, 2004. 30(7): p. 1348-55.
2. de Lassence, A., et al., Impact of unplanned extubation and reintubation after weaning on nosocomial pneumonia risk in the intensive care unit: a prospective multicenter stdy. Anesthesiology, 2002. 97(1): p. 148-56.
3. Medicine, S.f.C.C. Critical Care Statistics. 2017; Available from: http://www.sccm.org/Communications/Pages/CriticalCareStats.aspx.
4. Wunsch, H., et al., ICU occupancy and mechanical ventilator use in the United States. Crit Care Med, 2013. 41(12): p. 2712-9.
5. Needham, D.M. and P.J. Pronovost, The importance of understanding the costs of critical care and mechanical ventilation. Crit Care Med, 2005. 33(6): p. 1434-5.
6. Fisher, D.F., et al., Comparison of commercial and noncommercial endotracheal tube-securing devices. Respir Care, 2014. 59(9): p. 1315-23.
Notices:
Descriptions and discussions, if any, of properties, personnel, specific business plans, anticipated future results of operations and financial condition and other material information are not included in full, and the descriptions of the business and operations contained in this profile are brief. Nothing contained herein is, or should be relied on as, a promise, guarantee or representation as to the present or future performance of the company or its products. The technology and/or product described in this overview have not received market clearance by the FDA and are not for sale in the United Sales. Descriptions of product attributes and benefits are not intended to be marketing claims, but are set forth to assist in the evaluation of the potential risks and benefits of the technology should FDA clearance be secured. This summary does not constitute an offer to sell securities. Any and all such offers will be made only on an individual basis by an authorized officer or agent of Securisyn Medical.